Abstract
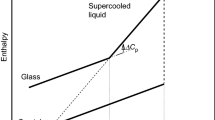
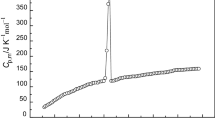
Glass transition temperature is one of the main criteria for the evaluation of the potential options for electrolyte application. Originally, in this communication, the attention was focused on the prediction of glass transition temperature of 1,3-dialkyl imidazolium ionic liquids which could be considered as potential future electrolytes. For this purpose, the quantitative structure–property relationship (QSPR) method is employed to develop two models for the determination of glass transition temperature. In part I of this study, genetic function approximation is applied for model’s parameter selection (molecular descriptors) and developing a linear QSPR model. The linear model tested by several validation techniques, and its stability is completely approved. The model predicts the experimental values with satisfactory results quantified by following statistical parameters: absolute average deviations (AAD): 2.68%, squared correlation coefficient: 0.91 and root mean square: 5.717 K.


Similar content being viewed by others
References
Jagadeeswara Rao C, Venkata Krishnan R, Venkatesan KA, Nagarajan K, Srinivasan TG. Thermochemical properties of some bis(trifluoromethyl-sulfonyl)imide based room temperature ionic liquids. J Therm Anal Calorim. 2009;97(3):937–43.
Murillo-Hernández JA, López-Ramírez S, Domínguez JM, Duran-Valencia C, García-Cruz I, González-Guevara JA. Survey on ionic liquids effect based on metal anions over the thermal stability of heavy oil. J Therm Anal Calorim. 2009;95(1):173–9.
Andrade CKZ, Matos RAF, Oliveira VB, Duraes JA, Sales MJA. Thermal study and evaluation of new menthol-based ionic liquids as polymeric additives. J Therm Anal Calorim. 2010;99(2):539–43.
Stefan CS, Lemordant D, Biensan P, Siret C, Claude-Montigny B. Thermal stability and crystallization of N-alkyl-N-alkyl-pyrrolidinium imides. J Therm Anal Calorim. 2010;102(2):685–93.
Zhang ZH, Cui T, Zhang JL, Xiong H, Li GP, Sun LX, et al. Thermodynamic investigation of room temperature ionic liquid: the heat capacity and thermodynamic functions of BMIPF6. J Therm Anal Calorim. 2010;101(3):1143–8.
Amarasekara AS, Owereh OS. Thermal properties of sulfonic acid group functionalized Brönsted acidic ionic liquids. J Therm Anal Calorim. 2011;103(3):1027–30.
Arellano IHJ, Guarino JG, Paredes FU, Arco SD. Thermal stability and moisture uptake of 1-alkyl-3-methylimidazolium bromide. J Therm Anal Calorim. 2011;103(2):725–30.
Keating MY, Gao F, Ramsey JB. TGA-MS study of the decomposition of phosphorus-containing ionic liquids trihexyl(tetradecyl)phosphonium decanoate and trihexyltetradecylphosphonium bis[(trifluoromethyl)sulfonyl] amide. J Therm Anal Calorim. 2011;106(1):207–11.
Porcedda S, Marongiu B, Schirru M, Falconieri D, Piras A. Excess enthalpy and excess volume for binary systems of two ionic liquids + water. J Therm Anal Calorim. 2011;103(1):29–33.
Yue D, Jing Y, Ma J, Yao Y, Jia Y. Physicochemical properties of ionic liquid analogue containing magnesium chloride as temperature and composition dependence. J Therm Anal Calorim. 2011:1–8.
Ohno H. Electrolytes ionic liquids. In: Jürgen G, editor. Encyclopedia of electrochemical power sources. Amsterdam: Elsevier; 2009. p. 153–9.
Manuel Stephan A. Review on gel polymer electrolytes for lithium batteries. Eur Polym J. 2006;42(1):21–42. doi:10.1016/j.eurpolymj.2005.09.017.
Gray FM. Solid polymer electrolytes: fundamentals and technological applications. New York: VCH; 1991.
Meindersma GW, Maase M, De Haan AB. Ionic liquids, Ullmann’s encyclopedia of industrial chemistry. Weinham: Wiley-VCH Verlag GmbH & Co. KGaA; 2000.
Schalkwijk WAv, Scrosati B. Advances in lithium-ion batteries. New York: Kluwer Academic/Plenum; 2002.
Angell CA. Glass transition. In: Buschow KHJ, Robert WC, Merton CF, Bernard I, Edward JK, Subhash M, et al., editors. Encyclopedia of materials: science and technology. Oxford: Elsevier; 2004. p. 1–11.
Freemantle M. An introduction to ionic liquids. Cambridge, MA: Royal Society of Chemistry; 2010. p. 2–3.
Gharagheizi F. QSPR studies for solubility parameter by means of genetic algorithm-based multivariate linear regression and generalized regression neural network. QSAR Comb Sci. 2008;27(2):165–70.
Vatani A, Mehrpooya M, Gharagheizi F. Prediction of standard enthalpy of formation by a QSPR model. Int J Mol Sci. 2007;8(5):407–32.
Gharagheizi F, Sattari M, Tirandazi B. Prediction of crystal lattice energy using enthalpy of sublimation: A group contribution-based model. Ind Eng Chem Res. 2011;50(4):2482–6.
Gharagheizi F. QSPR analysis for intrinsic viscosity of polymer solutions by means of GA-MLR and RBFNN. Comput Mater Sci. 2007;40(1):159–67.
Gharagheizi F. Quantitative structure–property relationship for prediction of the lower flammability limit of pure compounds. Energy Fuels. 2008;22(5):3037–9.
Gharagheizi F. A QSPR model for estimation of lower flammability limit temperature of pure compounds based on molecular structure. J Hazard Mater. 2009;169(1–3):217–20.
Gharagheizi F, Eslamimanesh A, Mohammadi AH, Richon D. QSPR approach for determination of parachor of non-electrolyte organic compounds. Chem Eng Sci. 2011;66(13):2959–67.
Gharagheizi F, Gohar MRS, Vayeghan MG. A quantitative structure–property relationship for determination of enthalpy of fusion of pure compounds. J Therm Anal Calorim. 2011:1–6.
Gharagheizi F, Babaie O, Mazdeyasna S. Prediction of vaporization enthalpy of pure compounds using a group contribution-based method. Ind Eng Chem Res. 2011;50(10):6503-7.
Gharagheizi F, Tirandazi B, Barzin R. Estimation of aniline point temperature of pure hydrocarbons: a quantitative structure–property relationship approach. Ind Eng Chem Res. 2009;48(3):1678–82.
Bonhôte P, Dias A-P, Papageorgiou N, Kalyanasundaram K, Grätzel M. Hydrophobic, highly conductive ambient-temperature molten salts. Inorg Chem. 1996;35(5):1168–78. doi:10.1021/ic951325x.
Branco LC, Rosa JN, Moura Ramos JJ, Afonso CAM. Preparation and characterization of new room temperature ionic liquids. Chem A Eur J. 2002;8(16):3671–7. doi:10.1002/1521-3765(20020816)8:16<3671:AID-chem3671>3.0.co;2-9.
Cha EH, Lim SA, Park JH, Kim DW, Macfarlane DR. Ionic conductivity studies of gel polyelectrolyte based on ionic liquid. J Power Sources. 2008;178(2):779–82. doi:10.1016/j.jpowsour.2007.10.033.
Chun S, Dzyuba SV, Bartsch RA. Influence of structural variation in room-temperature ionic liquids on the selectivity and efficiency of competitive alkali metal Salt extraction by a crown ether. Anal Chem. 2001;73(15):3737–41. doi:10.1021/ac010061v.
Crosthwaite JM, Muldoon MJ, Dixon JK, Anderson JL, Brennecke JF. Phase transition and decomposition temperatures, heat capacities and viscosities of pyridinium ionic liquids. J Chem Thermodyn. 2005;37(6):559–68. doi:10.1016/j.jct.2005.03.013.
D. Holbrey J, R. Seddon K. The phase behaviour of 1-alkyl-3-methylimidazolium tetrafluoroborates; ionic liquids and ionic liquid crystals. J Chem Soc Dalton Trans. 1999(13):2133–40.
Domańska U, Bogel-Łukasik E, Bogel-Łukasik R. 1-Octanol/water partition coefficients of 1alkyl-3-methylimidazolium chloride. Chem A Eur J. 2003;9(13):3033–41. doi:10.1002/chem.200204516.
Dzyuba SV, Bartsch RA. Influence of structural variations in 1-alkyl(aralkyl)-3-methylimidazolium hexafluorophosphates and bis(trifluoromethylsulfonyl)imides on physical properties of the ionic liquids. ChemPhysChem. 2002;3(2):161–6. doi:10.1002/1439-7641(20020215)3:2<161:aid-cphc161>3.0.co;2-3.
Dzyuba SV, Bartsch RA. Expanding the polarity range of ionic liquids. Tetrahedron Lett. 2002;43(26):4657–9. doi:10.1016/s0040-4039(02)00858-4.
Fredlake CP, Crosthwaite JM, Hert DG, Aki SNVK, Brennecke JF. Thermophysical properties of imidazolium-based ionic liquids. J Chem Eng Data. 2004;49(4):954–64. doi:10.1021/je034261a.
Fukumoto K, Yoshizawa M, Ohno H. Room temperature ionic liquids from 20 natural amino acids. J Am Chem Soc. 2005;127(8):2398–9. doi:10.1021/ja043451i.
Holbrey JD, Turner MB, Reichert WM, Rogers RD. New ionic liquids containing an appended hydroxyl functionality from the atom-efficient, one-pot reaction of 1-methylimidazole and acid with propylene oxide. Green Chem. 2003;5(6):731. doi:10.1039/b311717k.
Huddleston JG, Visser AE, Reichert WM, Willauer HD, Broker GA, Rogers RD. Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation. Green Chem. 2001;3(4):156–64. doi:10.1039/b103275p.
J. Earle M, B. McCormac P, R. Seddon K. Regioselective alkylation in ionic liquids. Chem Commun. 1998(20):2245–6.
Jodry JJ, Mikami K. New chiral imidazolium ionic liquids: 3D-network of hydrogen bonding. Tetrahedron Lett. 2004;45(23):4429–31. doi:10.1016/j.tetlet.2004.04.063.
Kulkarni PS, Branco LC, Crespo JG, Nunes MC, Raymundo A, Afonso CAM. Comparison of physicochemical properties of new ionic liquids based on imidazolium, quaternary ammonium, and guanidinium cations. Chem A Eur J. 2007;13(30):8478–88. doi:10.1002/chem.200700965.
MacFarlane DR, Golding J, Forsyth S, Forsyth M, Deacon GB. Low viscosity ionic liquids based on organic salts of the dicyanamide anion. Chem Commun. 2001;16:1430–1.
Matsumoto K, Hagiwara R. A new room temperature ionic liquid of oxyfluorometallate anion: 1-ethyl-3-methylimidazolium oxypentafluorotungstate (EMImWOF5). J Fluor Chem. 2005;126(7):1095–100. doi:10.1016/j.jfluchem.2005.03.018.
Matsumoto K, Hagiwara R, Ito Y. Room temperature molten fluorometallates: 1-ethyl-3-methylimidazolium hexafluoroniobate(V) and hexafluorotantalate(V). J Fluor Chem. 2002;115(2):133–5. doi:10.1016/s0022-1139(02)00037-4.
McEwen AB, Ngo HL, LeCompte K, Goldman JL. Electrochemical properties of imidazolium salt electrolytes for electrochemical capacitor applications. J Electrochem Soc. 1999;146(5):1687–95. doi:10.1149/1.1391827.
Moura Ramos J, Afonso C, Branco L. Glass transition relaxation and fragility in two room temperature ionic liquids. J Therm Anal Calorim. 2003;71(2):659–66. doi:10.1023/a:1022884716750.
Mu ZG, Zhou F, Zhang SX, Liang YM, Liu WM. Preparation and characterization of new phosphonyl-substituted imidazolium ionic liquids. Helvetica Chimica Acta. 2004;87(10):2549–55. doi:10.1002/hlca.200490227.
Ngo HL, LeCompte K, Hargens L, McEwen AB. Thermal properties of imidazolium ionic liquids. Thermochim Acta. 2000;357–358:97–102. doi:10.1016/s0040-6031(00)00373-7.
Nishida T. Physical and electrochemical properties of 1-alkyl-3-methylimidazolium tetrafluoroborate for electrolyte. J Fluor Chem. 2003;120(2):135–41. doi:10.1016/s0022-1139(02)00322-6.
Noda A, Hayamizu K, Watanabe M. Pulsed-gradient spin−echo 1H and 19F NMR ionic diffusion coefficient, viscosity, and ionic conductivity of non-chloroaluminate room-temperature ionic liquids. J Phys Chem B. 2001;105(20):4603–10. doi:10.1021/jp004132q.
Ogihara W, Washiro S, Nakajima H, Ohno H. Effect of cation structure on the electrochemical and thermal properties of ion conductive polymers obtained from polymerizable ionic liquids. Electrochim Acta. 2006;51(13):2614–9. doi:10.1016/j.electacta.2005.07.043.
Ohno H. Ion conductive characteristics of ionic liquids prepared by neutralization of alkylimidazoles. Solid State Ionics. 2002;154–155:303–9. doi:10.1016/s0167-2738(02)00526-x.
Omotowa BA, Shreeve JnM. Triazine-based polyfluorinated triquaternary liquid salts: synthesis, characterization, and application as solvents in rhodium(I)-catalyzed hydroformylation of 1-octene. Organometallics. 2004;23(4):783–91. doi:10.1021/om0342311.
Ouadi A, Gadenne B, Hesemann P, Moreau JJE, Billard I, Gaillard C, et al. Task-specific ionic liquids bearing 2-hydroxybenzylamine units: synthesis and americium-extraction studies. Chem A Eur J. 2006;12(11):3074–81. doi:10.1002/chem.200500741.
Park HS, Choi YS, Jung YM, Hong WH. Intermolecular interaction-induced hierarchical transformation in 1D nanohybrids: analysis of conformational changes by 2D correlation spectroscopy. J Am Chem Soc. 2007;130(3):845–52. doi:10.1021/ja073074k.
Pringle JM, Golding J, Baranyai K, Forsyth CM, Deacon GB, Scott JL, et al. The effect of anion fluorination in ionic liquids-physical properties of a range of bis(methanesulfonyl)amide salts. New J Chem. 2003;27(10):1504–10.
Qi M, Wu G, Li Q, Luo Y. γ-Radiation effect on ionic liquid [bmim][BF4]. Radiat Phys chem. 2008;77(7):877–83. doi:10.1016/j.radphyschem.2007.12.007.
Sato T. Electrochemical properties of novel ionic liquids for electric double layer capacitor applications. Electrochimica Acta. 2004;49(21):3603–11. doi:10.1016/j.electacta.2004.03.030.
Song Y, Liu L, Zhu X, Wang X, Jia H, Xiao X, et al. Physicochemical properties of ionic liquids based on imidazolium/pyrrolidinium cations and maleate/phthalate anions. Solid State Ionics. 2008;179(13–14):516–21. doi:10.1016/j.ssi.2008.03.035.
Strechan AA, Paulechka YU, Blokhin AV, Kabo GJ. Low-temperature heat capacity of hydrophilic ionic liquids [BMIM][CF3COO] and [BMIM][CH3COO] and a correlation scheme for estimation of heat capacity of ionic liquids. J Chem Thermodyn. 2008;40(4):632–9. doi:10.1016/j.jct.2007.11.004.
Suarez PAZ, Dullius JEL, Einloft S, De Souza RF, Dupont J. The use of new ionic liquids in two-phase catalytic hydrogenation reaction by rhodium complexes. Polyhedron. 1996;15(7):1217–9. doi:10.1016/0277-5387(95)00365-7.
Suarez PAZ, Einloft S, Dullius JEL, De Souza RF, Dupont J. Synthesis and physical-chemical properties of ionic liquids based on 1-n-butyl-3-methylimidazolium cation. Journal de Chimie Physique et de Physico-Chimie Biologique. 1998;95(7):1626–39.
Tosoni M, Laschat S, Baro A. Synthesis of novel chiral ionic liquids and their phase behavior in mixtures with smectic and nematic liquid crystals. Helvetica Chimica Acta. 2004;87(11):2742–9. doi:10.1002/hlca.200490247.
Valkenburg M, Vaughn R, Williams M, Wilkes J. Thermochemistry of ionic liquid heat-transfer fluids. Thermochim Acta. 2005;425(1–2):181–8. doi:10.1016/j.tca.2004.11.013.
Visser Ann E, Reichert WM, Swatloski Richard P, Willauer Heather D, Huddleston Jonathan G, Rogers Robin D. Characterization of hydrophilic and hydrophobic ionic liquids: alternatives to volatile organic compounds for liquid–liquid separations. Ionic liquids. ACS symposium Series 818. Washington DC: American Chemical Society; 2002. p. 289–308.
Xu W, Wang L-M, Nieman RA, Angell CA. Ionic liquids of chelated orthoborates as model ionic glassformers. J Phys Chem B. 2003;107(42):11749–56. doi:10.1021/jp034548e.
Yagupolskii YL, Sokolenko TM, Petko KI, Yagupolskii LM. Novel ionic liquids—Imidazolium salts with a difluoromethylene fragment directly bonded to the nitrogen atom. J Fluor Chem. 2005;126(4):669–72.
Ye C, Shreeve JNM. Syntheses of very dense halogenated liquids. J Org Chem. 2004;69(19):6511–3. doi:10.1021/jo0490649.
Yeon S, Kim K, Choi S, Lee H, Kim H. Physical and electrochemical properties of 1-(2-hydroxyethyl)-3-methyl imidazolium and N-(2-hydroxyethyl)-N-methyl morpholinium ionic liquids. Electrochimica Acta. 2005;50(27):5399–407. doi:10.1016/j.electacta.2005.03.020.
Yoshida Y, Muroi K, Otsuka A, Saito G, Takahashi M, Yoko T. 1-Ethyl-3-methylimidazolium based ionic liquids containing cyano groups: synthesis, characterization, and crystal structure. Inorg Chem. 2004;43(4):1458–62. doi:10.1021/ic035045q.
Zhou Z-B, Matsumoto H, Tatsumi K. Low-melting, low-viscous, hydrophobic ionic liquids: 1-alkyl(alkyl ether)-3-methylimidazolium perfluoroalkyltrifluoroborate. Chem A Eur J. 2004;10(24):6581–91. doi:10.1002/chem.200400533.
Zhou Z-B, Matsumoto H, Tatsumi K. Structure and properties of new ionic liquids based on alkyl- and alkenyltrifluoroborates. ChemPhysChem. 2005;6(7):1324–32. doi:10.1002/cphc.200500094.
Rogers D, Hopfinger AJ. Application of genetic function approximation to quantitative structure-activity relationships and quantitative structure–property relationships. J Chem Inf Comput Sci. 1994;34(4):854–66.
Friedman JH. Multivariate adaptive regression splines. Ann Stat. 1991;19(1):1–67. doi:10.1214/aos/1176347963.
Holland JH. Adaptation in natural and artificial systems: an introductory analysis with applications to biology, control, and artificial intelligence. 1st ed. Cambridge, MA: MIT Press; 1992.
Ahmed SSSJ, Ahameethunisa A, Santosh W. QSAR and pharmacophore modeling of 4-arylthieno [3, 2-d] pyrimidine derivatives against adenosine receptor of Parkinson’s disease. J Theor Comput Chem. 2010;9(6):975–91.
Chhabria MT, Jani M, Parmar K, Singh M. QSAR study of a series of 2,3-dihydroimidazo[1,2-c]pyrimidines as antibacterial agents. Medicinal Chemistry Research. 2010:1–8.
Chhabria MT, Suhagia BN, Mandhare AB, Brahmkshatriya PS. QSAR study of a series of cholesteryl ester transfer protein inhibitors. Collect Czech Chem Commun. 2011;76(7):803–13.
Khaled KF. Modeling corrosion inhibition of iron in acid medium by genetic function approximation method: a QSAR model. Corros Sci. 2011;53(11):3457–65.
Ojha PK, Roy K. Chemometric modelling of antimalarial activity of aryltriazolylhydroxamates. Mol Simul. 2010;36(12):939–52.
Reyes OJ, Patel SJ, Mannan MS. Quantitative structure property relationship studies for predicting dust explosibility characteristics (Kst, Pmax) of organic chemical dusts. Ind Eng Chem Res. 2011;50(4):2373–9.
Sivakumar PM, Iyer G, Doble M. QSAR studies on substituted 3- or 4-phenyl-1,8-naphthyridine derivatives as antimicrobial agents. Med Chem Res. 2011:1–8.
Gharagheizi F. An accurate model for prediction of autoignition temperature of pure compounds. J Hazard Mater. 2011;189(1–2):211–21.
Liu H, Yao X, Zhang R, Liu M, Hu Z, Fan B. Accurate quantitative structure–property relationship model to predict the solubility of C60 in various solvents based on a novel approach using a least-squares support vector machine. J Phys Chem B. 2005;109(43):20565–71.
Burden FR. Molecular identification number for substructure searches. J Chem Inf Comput Sci. 1989;29(3):225–7. doi:10.1021/ci00063a011.
Pearlman RS, Smith KM. Metric validation and the receptor-relevant subspace concept. J Chem Inf Comput Sci. 1999;39(1):28–35. doi:10.1021/ci980137x.
Gao H. Application of BCUT metrics and genetic algorithm in binary QSAR analysis. J Chem Inf Comput Sci. 2001;41(2):402–7. doi:10.1021/ci000306p.
Stanton DT. Evaluation and use of BCUT descriptors in QSAR and QSPR studies. J Chem Inf Comput Sci. 1998;39(1):11–20. doi:10.1021/ci980102x.
Todeschini R, Consonni V. Molecular descriptors for chemoinformatics. 2nd ed. Weinheim: Wiley-VCH; 2009.
Krzanowski WJ. Principles of multivariate analysis: a user’s perspective. Oxford: Oxford University Press; 2000.
Todeschini R, Consonni V, Mauri A, Pavan M. Detecting “bad” regression models: multicriteria fitness functions in regression analysis. Analytica Chimica Acta. 2004;515(1):199–208. doi:10.1016/j.aca.2003.12.010.
Chiou J. Hybrid method of evolutionary algorithms for static and dynamic optimization problems with application to a fed-batch fermentation process. Comput Chem Eng. 1999;23(9):1277–91. doi:10.1016/s0098-1354(99)00290-2.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mousavisafavi, S.M., Mirkhani, S.A., Gharagheizi, F. et al. A predictive quantitative structure–property relationship for glass transition temperature of 1,3-dialkyl imidazolium ionic liquids. J Therm Anal Calorim 111, 235–246 (2013). https://doi.org/10.1007/s10973-012-2207-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2207-8